TARGIT - IORT

Intrabeam TARGIT IORT is targeted intraoperative radiotherapy treatment for breast cancer. Conceived in the UK more than 20 years ago, thoroughly tested in clinical trials, TARGIT IORT treatment is widely used to treat breast cancer patients across the world.
“The most important benefit of TARGIT for a woman with breast cancer is that it allows her to complete her entire local treatment [lumpectomy and radiation therapy] at the time of her operation, with lower toxicity.” Lancet 2014;383:603-613
In 2021, TARGIT-IORT was included among the "Five amazing health research breakthroughs .." by National Institute for Health Research, NIHR, UK, alongside the Oxford COVID vaccine and two COVID treatments.
Please visit the dedicated website for TARGIT-IORT
Special relevance of TARGIT-IORT during the COVID-19 pandemic
TARGIT-IORT is given as a single dose during the cancer operation - so in most patients no visits to the radiotherapy department are required. This is especially relevant during the COVID-19 pandemic. When the radiotherapy is completed during the cancer operation, under the same anaesthetic, it greatly reduces the need for repeated post-operative visits to the hospital for postoperative external beam radiotherapy which are necessary for regimens such as IMPORT-Low or Fast-Forward which irradiates the whole breast.
This is why it has been included in several national and international guidelines for treatment of breast cancer during the COVID-19 pandemic caused by the widespread infection with the SARS-CoV-2 virus
During the COVID-19 pandemic, new guidelines for treatment of beast cancer and radiotherapy have recommend using TARGIT-IORT because it would save patients the daily travel (and viral exposure) to radiotherapy departments:
Global radiation oncology's targeted preparedness for COVID-19
https://www.sciencedirect.com/science/article/pii/S2405630820300227
“where the technology is available the use of IORT may obviate the need for any further outpatient treatment and should be considered an option ..”
British Association of Cancer Surgery (BASO~ACS) https://baso.org.uk/media/98159/covid_19_and_breast_cancer_march_2020.pdf
German University Hospitals
https://ro-journal.biomedcentral.com/articles/10.1186/s13014-020-01527-1
Italian Association of Radiotherapy and Clinical Oncology
https://www.thegreenjournal.com/article/S0167-8140(20)30221-8/fulltext
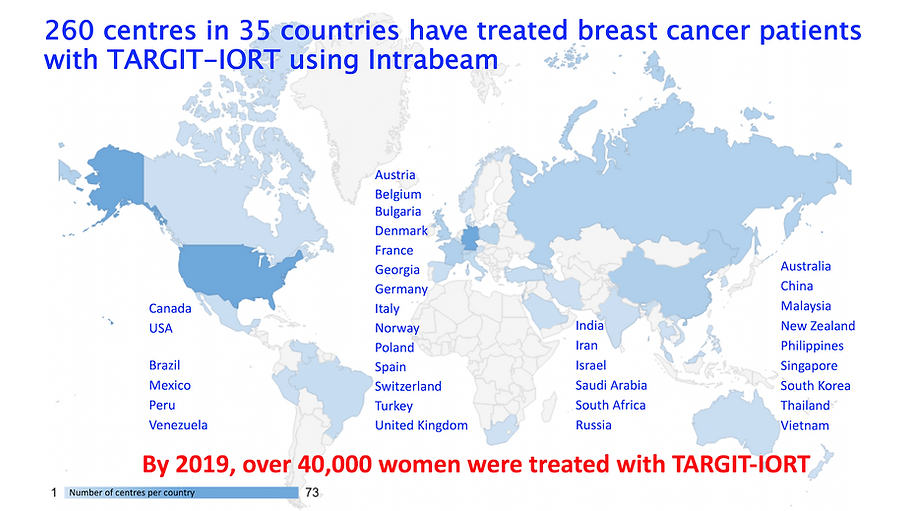
China is increasing its use of TARGIT-IORT #breastcancer by equipping more centres with the TARGIT-IORT equipment
Click here to see the national and international guidelines recommending TARGIT-IORT for breast cancer
Information about Intrabeam TARGIT IORT
Intrabeam TARGIT IORT for breast cancer is clinically effective - confirmed in the TARGIT-A international clinical trial

First publication of the main results (2010)
Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial.
The Lancet. 2010 ;376(9735):91-102.
5-year results and first analysis of survival (2014)
Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial.
The Lancet. 11 November 2013. doi:10.1016/s0140-6736(13)61950-9
Full 226 page report of the TARGIT-A trial of Intrabeam TARGIT IORT for HTA, NIHR, Department of Health, UK (2016)
An international randomised controlled trial to compare TARGeted Intraoperative radioTherapy (TARGIT) with conventional postoperative radiotherapy after breast-conserving surgery for women with early-stage breast cancer (the TARGIT-A trial).
Health Technology Assessment 2016;20(73)
Long term outcomes of the TARGIT-A trial (2020)
Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial
BMJ British Medical Journal 2020; 370 doi: https://doi.org/10.1136/bmj.m2836 (19 August 2020)
New clinical and biological findings from the long-term follow up of TARGIT-A trial (2021)
New clinical and biological insights from the international TARGIT-A randomised trial of targeted intraoperative radiotherapy during lumpectomy for breast cancer
Global adoption of TARGIT-IORT (2022)
Global adoption of single-shot targeted intraoperative radiotherapy (TARGIT-IORT) for breast cancer—better for patients, better for healthcare systems
Frontiers in Oncology 2022; 12: https://doi.org/10.3389/fonc.2022.786515
Results of the TARGIT-A trial in the top Radiotherapy Journal - The Red Journal
The TARGIT-A Randomized Trial: TARGIT-IORT Versus Whole Breast Radiation Therapy: Long-Term Local Control and Survival
International Journal of Radiation Oncology Biology Physics 2023; 115:77 82. https://doi.org/10.1016/j.ijrobp.2022.08.029
Recent peer reviewed publications are below: more are available here
-
Red Journal: Meta-analyis Improved survival with targeted radiotherapy
-
Lancet: Updated meta-analysis of targeted radiotherapy confirming the survival benefit
-
Lancet: A meta-analysis of the GEC-ESTRO and the TARGIT-A: 5 year data
-
BMJ Open: Environmental and Social benefits of Intrabeam TARGIT IORT
-
Red Journal: Better breast-related quality of life with TARGIT IORT
-
NIHR Journals: Cost-Utility analysis of TARGIT IORT - Saving £9 million/yr in thUK
-
BMJ Open: Health Economics of TARGIT-IORT: Less costly for NHS
-
Breast Care: A tumour bed boost after neoadjuvant chemotherapy
-
BMJ Evidence Based Medicine: commentary on the IMPORT-LOW trial
-
PRO: Commentary and corrections to the 2017 ASTRO APBI consensus
Surgical technique video of TARGIT IORT for breast cancer
iPhone App for helping to choose TARGIT for appropriate breast cancer patients. Android version



